Introduction
Acute promyelocytic leukemia (APL), considered a medical emergency, requires prompt treatment at the first suspicion of the diagnosis. Treatment is initiated even before cytogenetic or molecular confirmation of diagnosis because of high early mortality with APL, mostly due to hemorrhage and disseminated intravascular coagulation. Once a cancer with dismal prognosis, APL is now considered a highly curable cancer with the introduction of all-trans retinoic acid and arsenic trioxide. In fact, recent clinical trials in APL report excellent outcomes with >90% OS at 3-years with <5% mortality in the first 4-6 weeks. Here we analyze a large National Cancer Database (NCDB) to determine one-month mortality and overall survival (OS) of patients with APL treated in real-world practices.
Methods
We utilized the NCDB Participant User File to identify patients of all age groups who were diagnosed between 2004 and 2015. A Chi-square test was used to determine association of different covariates with one-month mortality. Multiple logistic regression analysis was used to evaluate the effect of covariates on the probability of one-month mortality. A full model was run that included all covariates; the subsequent reduced model included covariates significant at the p<0.1. OS was analyzed with Kaplan Meier methods.
Results
Our study included a total of 7190 patients- median age was 50 years, 50% were female, 82% were white, 53% had private insurance, 82% had Charlson comorbidity index (CCI) of 0 or 1, and 57% were treated at academic centers. Ninety-seven percent of patients were treated with either multiagent (67%) or single-agent chemotherapy (30%). Patients diagnosed in 2004-2009 comprised 49% of total cohort, the remaining 51% were diagnosed in 2010-2015.
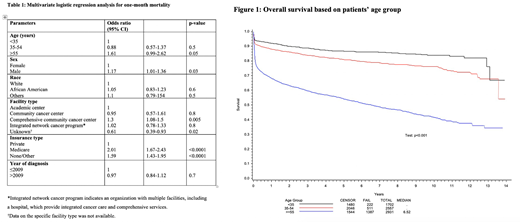
One-month mortality for the entire cohort was 12%. One-month mortality varied significantly for different age groups: 6% for <35, 8% for 35-54, and 18% for ≥55 years. One-month mortality was not significantly different based on the year of diagnosis or CCI. In a multivariate analysis, patients <55 years of age (p<0.0001), and those with private insurance (p<0.0001) had lower one-month mortality (Table 1). Patients treated at comprehensive cancer centers, as compared to academic centers, had worse one-month mortality (p=0.005).
Median OS at 1-, 3-, and 5-year were 81%, 75%, and 71% respectively. OS differed significantly based on age. Median OS was not reached for patients <55 years, compared to 6.5 years for patients ≥55 years (Figure 1). Detailed analysis will be presented.
Conclusion
Our large national database study demonstrates that early mortality is still high in real-world practices. OS at 1 year (81%) is worse than reported OS of >90% in clinical trials. Surprisingly, only 1/3rd of patients received single agent chemotherapy, which is not the standard of care. The use of suboptimal chemotherapy may not explain high early mortality rate but may explain worse OS. We also identified age as a significant predictor of outcomes with older adults ≥55 years almost at a two-fold higher risk of early and late mortality. Lower rate of enrollment of older adults may yet be another reason for higher OS seen in clinical trials. Our findings highlight the challenges associated with translating trial findings to clinical practices and provide rationale to support studies aimed at improving outcomes of APL in the real world.
Gundabolu:BioMarin: Consultancy; Bristol Myers Squibb pharmaceuticals: Consultancy. Chaulagain:Sanofi Genzyme: Honoraria. Bhatt:Tolero: Research Funding; Pfizer: Research Funding; Abbvie: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Jazz: Research Funding; Rigel: Consultancy; Oncoceutics: Other; National Marrow Donor Program: Research Funding; Partnership for health analytic research: Consultancy; Takeda: Consultancy; Omeros: Consultancy; Agios: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal